Intrathoracic Pressure Regulation (IPR) Devices
IPR therapy enhances negative intrathoracic pressure within the chest, which improves preload, cardiac output and lowers intracranial pressure (ICP) to provide better perfusion to the brain and vital organs.1 IPR therapy is currently delivered with ZOLL's ResQPOD® impedance threshold device (ITD), the ResQGARD® ITD and the ResQCPR® System.
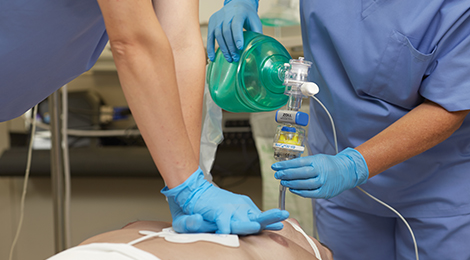
ResQPOD ITD
The ResQPOD ITD is a simple, non-invasive device that delivers IPR therapy during basic or advanced life support CPR to enhance perfusion. When used with high-quality CPR, the ResQPOD ITD has been shown in clinical studies to improve survival by 25% or more.2
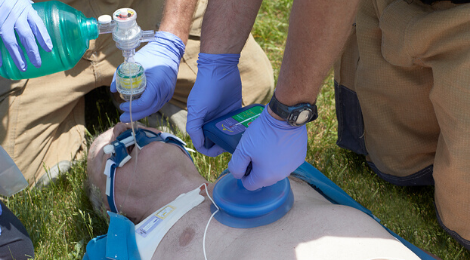
ResQCPR System
The ResQCPR System, consisting of the ResQPOD ITD and the ResQPUMP® ACD-CPR device, takes the perfusion enhancing-power of the ResQPOD ITD to the next level. The ResQPUMP allows the rescuer to perform active compression-decompression CPR (ACD-CPR), which promotes complete and active chest recoil. In a major clinical study, the ResQCPR System showed a 49% increase in one-year survival from cardiac arrest.* It is the only CPR device with an FDA-approved indication to improve the likelihood of survival.
1 Metzger A, Rees J, Segal N, McKnite S, Matsuura T, Convertino V, Gerhardt RT, Lurie KG. "Fluidless" resuscitation with permission hypotension via impedance threshold device therapy compared with normal saline resuscitation in a porcine model of severe hemorrhage. J Trauma Acute Care Surg 2013;75:S203-S209.
2Yannopoulos D, et al. Resuscitation. 2015;94:106-113.
*ResQCPR System Summary of Safety and Effectiveness Data submitted to FDA.