March 22, 2012 - ZOLL X Series Monitor Defibrillator Granted 510(K) Clearance by U.S. Food and Drug Administration
INVESTOR CONTACT: |
A. Ernest Whiton |
MEDIA CONTACT: |
Diane Egan |
ZOLL X SERIES MONITOR/DEFIBRILLATOR GRANTED
510(K) CLEARANCE BY U.S. FOOD AND DRUG ADMINISTRATION
New Small, Light, Powerful Device Designed for Emergency Medical Services
March 22, 2012─CHELMSFORD, MASS.–ZOLL Medical Corporation (Nasdaq GS: ZOLL), a manufacturer of medical devices and related software solutions, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration to market and begin U.S. 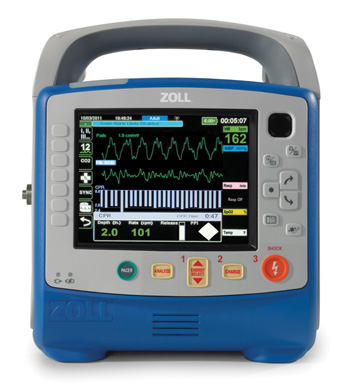
Weighing less than 12 pounds (6 kilograms), the X Series is compact without compromise in capability, performance, or display size. This monitor/defibrillator combines the clinically superior therapeutic capabilities of ZOLL defibrillation, pacing, and CPR assistance with advanced monitoring parameters.
“We believe the X Series is a game changer for ZOLL in the EMS market. We have the size and weight advantages that ZOLL is known for without compromising features or functionality,” said Jonathan A. Rennert, President of ZOLL. “The X Series has every advanced monitoring and communication capability required by EMS providers and, ultimately, critical care transport operations.”
“The X Series should be a ‘must see’ for any service interested in cutting edge technology. It was built on the Propaq® MD Monitor/Defibrillator platform, which in little more than a year has become a standard of care for air medical operations worldwide,” he added.
The large bright screen on the X Series allows for simultaneous viewing of up to four different waveform traces, as well as split-screen 12-lead views. The device incorporates Masimo rainbow® SET CO-Oximetry™ technology, which provides Masimo SET® SpO2, pulse rate, perfusion index (PI), carboxyhemoglobin (SpCO®), and methemoglin (SpMet®). Welch Allyn Non-invasive Blood Pressure (NIBP) technology─Sure BP® and Smart Cuf®─is yet another capability that sets the X Series apart from other defibrillators. Acknowledged as the best of class NIBP technology, it offers faster, more accurate readings.
For unequaled CPR support, the X Series features ZOLL’s full suite of CPR assist tools including Real CPR Help® for real-time CPR feedback and See-ThruCPR® artifact filtering to help minimize the length of pauses. It’s the first device to feature ZOLL’s CPR Dashboard™, which displays CPR quality measures in real time.
A major breakthrough in the X Series is its advanced communications capabilities. Leveraging a dedicated onboard data processor—essentially a smart phone in the box—the X Series is the first and only EMS monitor/defibrillator that can communicate via Bluetooth, WiFi, or USB cellular modem. Streaming data can be sent to hospitals or clinicians at remote locations while rescuers are caring for patients. For STEMI patients, the X Series is designed to speed door-to-balloon times with quick and accurate 12-lead acquisition, interpretation, and transmission. The X Series fully integrates with ZOLL’s RescueNet® 12-Lead and RescueNet Medgate Management Systems.
The X Series, along with the Propaq MD, are the only two defibrillators to earn an IP55 ingress protection rating for dust and water. Both units are virtually immune to a three-minute, high-pressure onslaught of water and meet the most stringent specifications for shock and vibration. The X Series is powered by a SurePower™ II high-capacity lithium-ion battery for six hours of continuous run time.
About ZOLL Medical Corporation
ZOLL Medical Corporation develops and markets medical devices and software solutions that help advance emergency care and save lives, while increasing clinical and operational efficiencies. With products for defibrillation and monitoring, circulation and CPR feedback, data management, fluid resuscitation, and therapeutic temperature management, ZOLL provides a comprehensive set of technologies that help clinicians, EMS and fire professionals, and lay rescuers treat victims needing resuscitation and critical care.
A NASDAQ Global Select company and a three-time Forbes 100 Most Trustworthy Company, ZOLL was designated in 2011 as one of Forbes Top 100 Small Public Companies in America with annual revenues under $1 billion. ZOLL develops and manufactures its products in the United States, in California, Colorado, Illinois, Massachusetts, Pennsylvania, and Rhode Island. More than 400 direct sales and service representatives, 1,100 business partners, and 200 independent representatives serve our customers in over 140 countries around the globe. For more information, visit www.zoll.com.
Certain statements contained in this press release, including statements regarding the anticipated development of the Company's business, and other statements contained herein regarding matters that are not historical facts, are “forward-looking” statements (as defined in the Private Securities Litigation Reform Act of 1995). Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause actual results to differ materially from those expressed or implied by such forward-looking statements include, but are not limited to, those factors discussed in the section entitled “Risk Factors” in the Company's Annual Report on Form 10-K filed with the SEC on November 23, 2011, as updated by the Company’s subsequent SEC filings, including Quarterly Reports on Form 10-Q. You should not place undue reliance on the forward-looking statements in this press release, and the Company disavows any obligation to update or supplement those statements in the event of any changes in the facts, circumstances, or expectations that underlie those statements.
Copyright © 2012 ZOLL Medical Corporation. All rights reserved. CPR Dashboard, Real CPR Help, RescueNet, See-Thru CPR, SurePower, X Series and ZOLL are trademarks and/or registered trademarks of ZOLL Medical Corporation in the United States and/or other countries. Masimo, SpCO, SpMet, Rainbow, and SET are registered trademarks of Masimo Corporation. All trademarks are the property of their respective owners.
